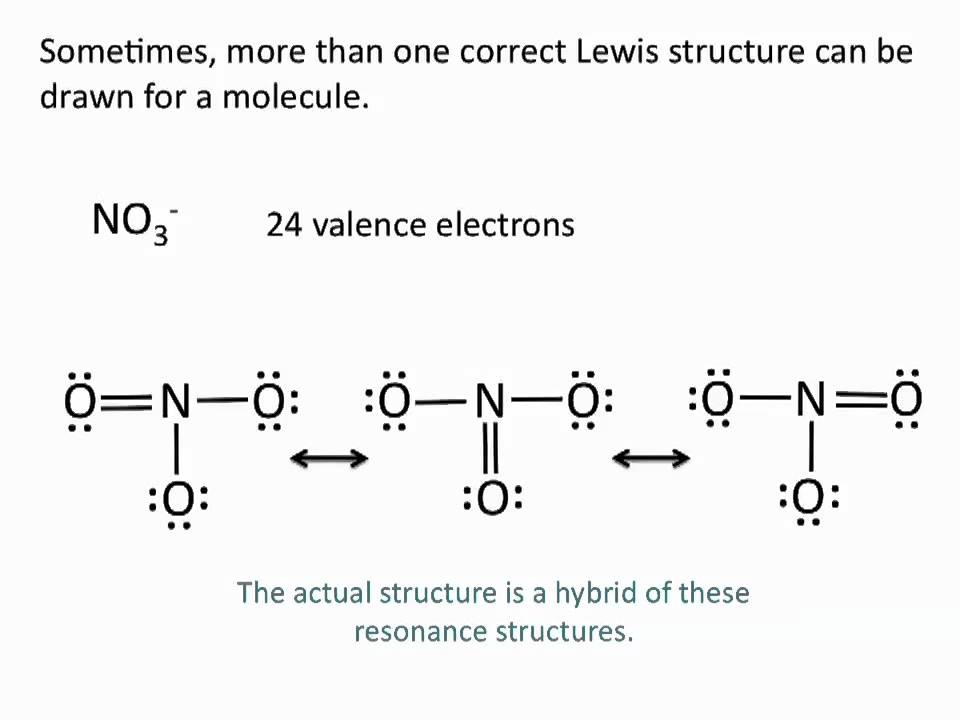
For Resonance Forms Of A Molecule Or Ion - A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single.
A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single.
In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
How to Know if a Molecule Has Resonance LeakruwBall
The actual structure is something in between the resonance structures and is known as a resonance hybrid. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single.
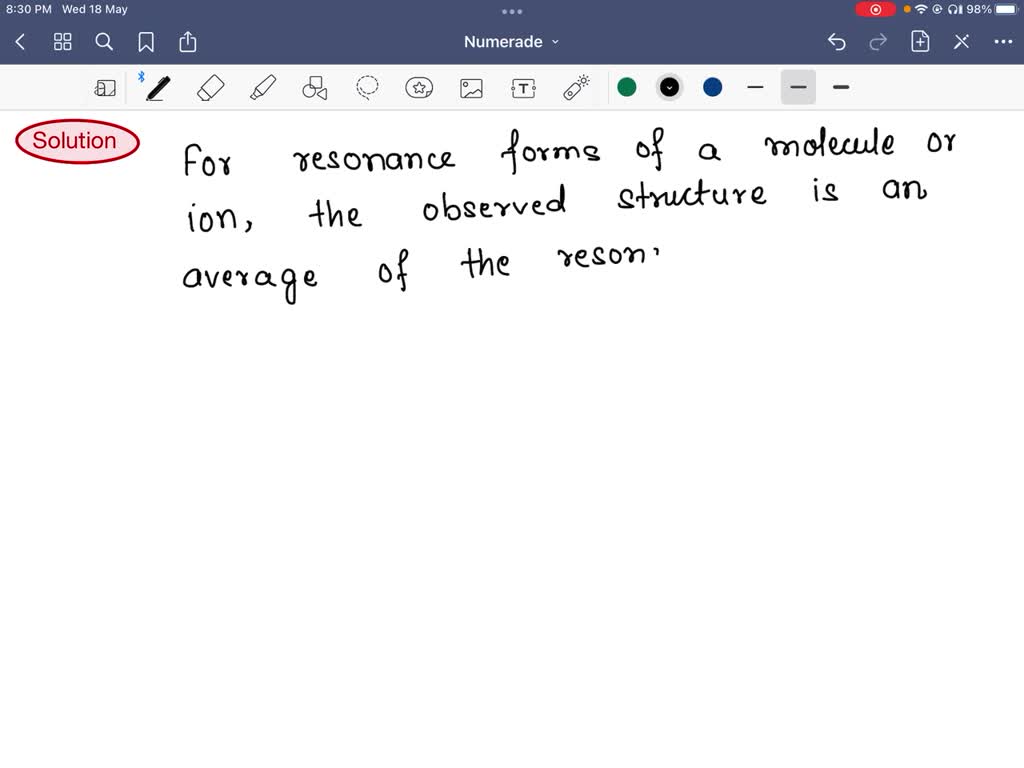
SOLVED For resonance forms of a molecule or ion, one always
In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
[Solved] When a molecule has resonance forms, the most stable forms
The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or.
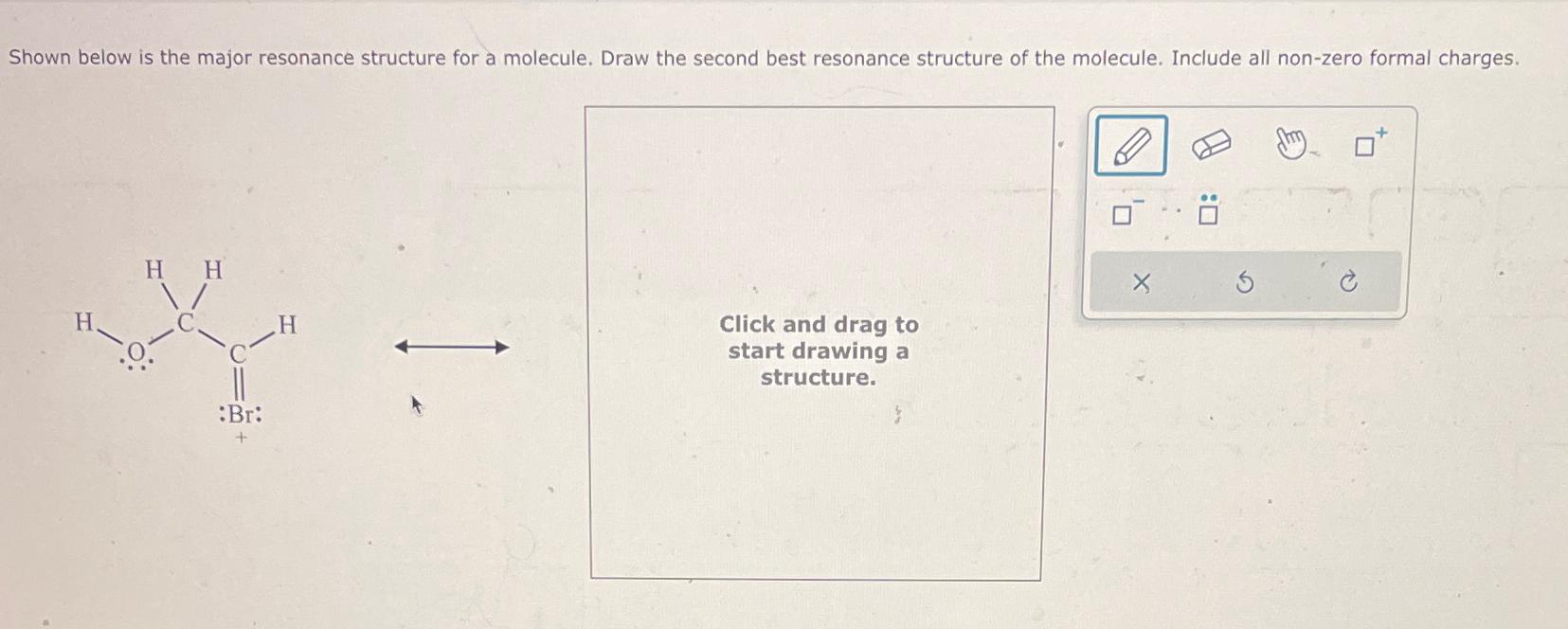
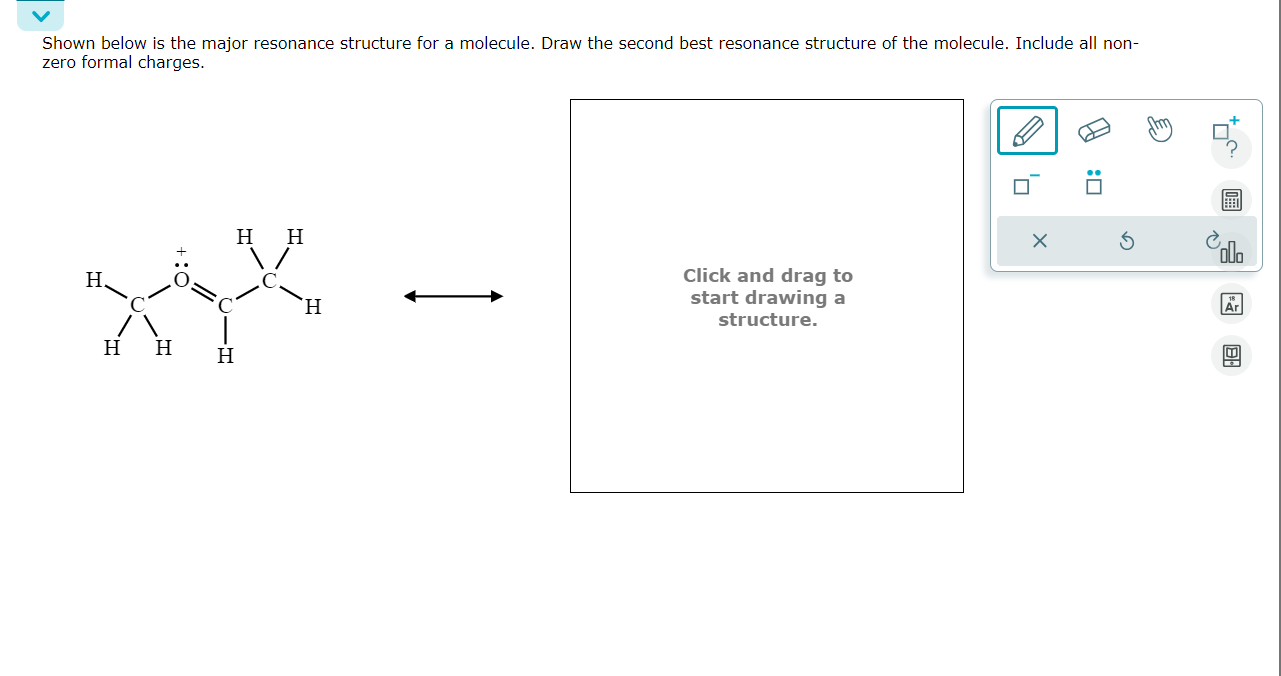
Solved Shown below is the major resonance structure for a
A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
[Solved] Resonance forms Many molecules have resonance forms in which
A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
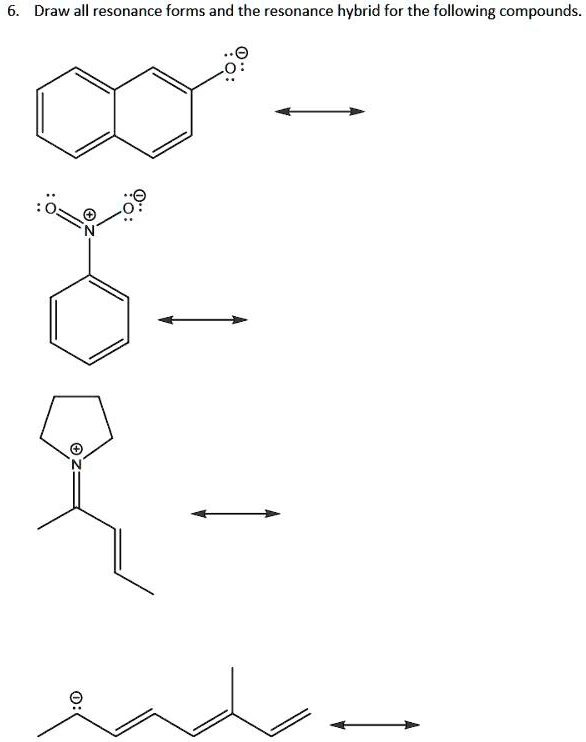
SOLVED Draw all resonance forms and the resonance hybrid for the
In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. The actual structure is something in between the resonance structures and is known as a resonance hybrid. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or.
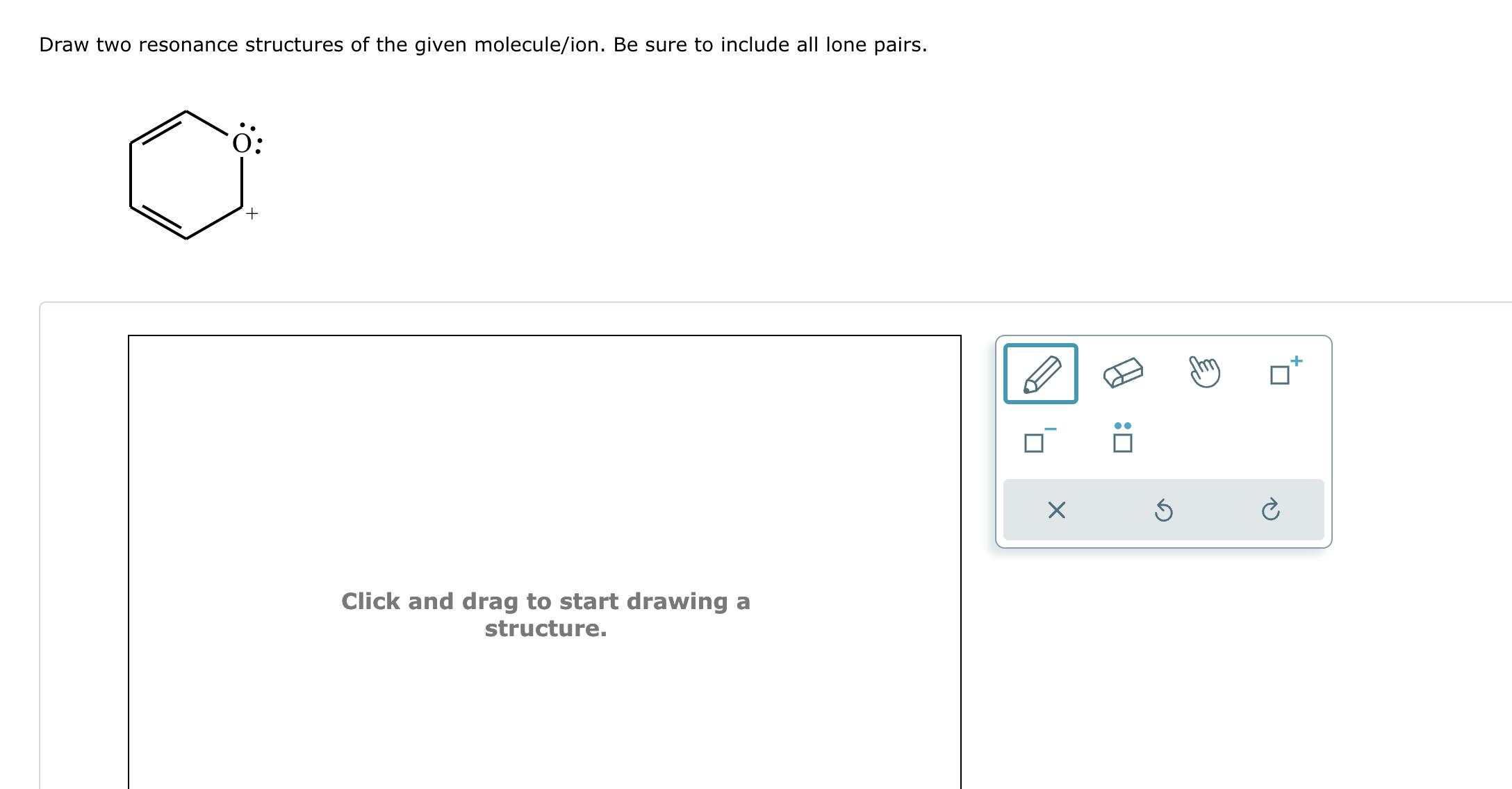
Solved Draw two resonance structures of the given
In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. The actual structure is something in between the resonance structures and is known as a resonance hybrid. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or.
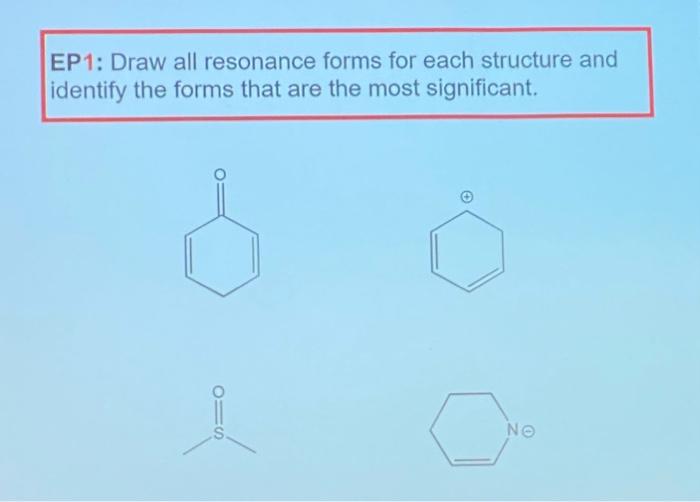
Solved EP1 Draw all resonance forms for each structure and
In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
Solved Shown below is the major resonance structure for a
A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single.
[Solved] Drawing resonance structure. Q2) Draw the MULTIPLE resonance
A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
A Molecule Or Ion With Such Delocalized Electrons Is Represented By Several Contributing Structures (Also Called Resonance Contributors Or.
In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. The actual structure is something in between the resonance structures and is known as a resonance hybrid.