Energy And Specific Heat Report Sheet - Why is this important for the human body? Equation 1 shows how to calculate specific heat. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. What is the specific heat of a substance? Heat is the energy transferred due to temperature differences between two different objects. Specific heat is unique to each substance and can be used to identify unknown substances. Q.2 water has one of the largest specific heats of any substance. Heat is the energy that is transferred from one body to another as a result of a difference in temperature to be able to reach thermal. The exact amount of heat q transferred due. What heat is needed to raise 3.4 kg of lead from 23 oc to 58 oc?
Q.2 water has one of the largest specific heats of any substance. What heat is needed to raise 3.4 kg of lead from 23 oc to 58 oc? Why is this important for the human body? Equation 1 shows how to calculate specific heat. The exact amount of heat q transferred due. Specific heat is unique to each substance and can be used to identify unknown substances. Heat is the energy transferred due to temperature differences between two different objects. What is the specific heat of a substance? Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Heat is the energy that is transferred from one body to another as a result of a difference in temperature to be able to reach thermal.
The exact amount of heat q transferred due. Heat is the energy that is transferred from one body to another as a result of a difference in temperature to be able to reach thermal. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. What heat is needed to raise 3.4 kg of lead from 23 oc to 58 oc? Heat is the energy transferred due to temperature differences between two different objects. Q.2 water has one of the largest specific heats of any substance. What is the specific heat of a substance? Equation 1 shows how to calculate specific heat. Why is this important for the human body? Specific heat is unique to each substance and can be used to identify unknown substances.
Heat And Heat Calculations Worksheets
Equation 1 shows how to calculate specific heat. Heat is the energy transferred due to temperature differences between two different objects. What heat is needed to raise 3.4 kg of lead from 23 oc to 58 oc? What is the specific heat of a substance? The exact amount of heat q transferred due.
Pages Reports Heat
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Heat is the energy that is transferred from one body to another as a result of a difference in temperature to be able to reach thermal. Heat is the energy transferred due to temperature differences.
Solved Specific Heat Report indicates the value you
The exact amount of heat q transferred due. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Heat is the energy transferred due to temperature differences between two different objects. Q.2 water has one of the largest specific heats of any substance. What is.
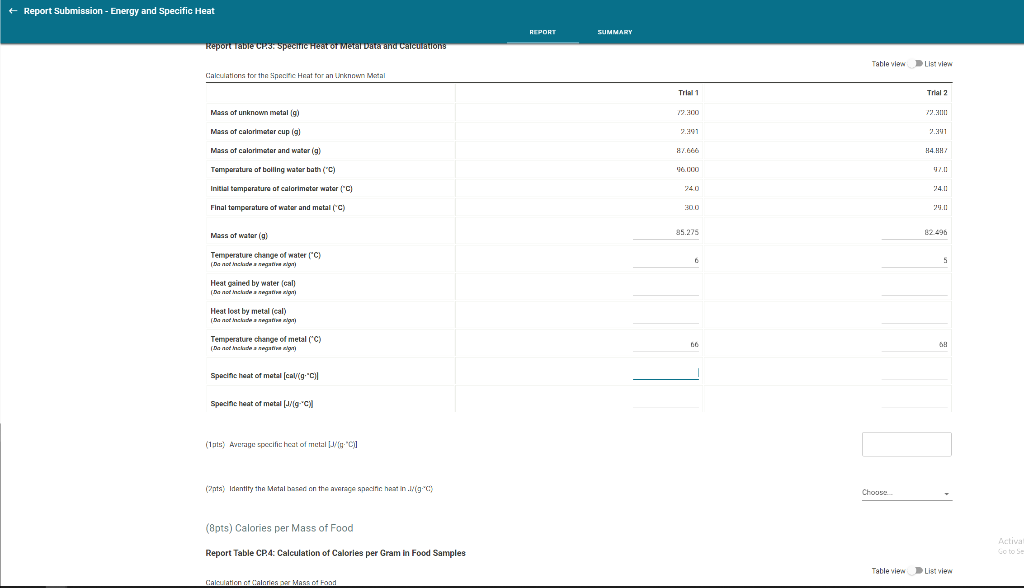
Solved Energy and Specific Heat Report Sheet A 10
Heat is the energy that is transferred from one body to another as a result of a difference in temperature to be able to reach thermal. What heat is needed to raise 3.4 kg of lead from 23 oc to 58 oc? What is the specific heat of a substance? Why is this important for the human body? Q.2 water.
Solved + Report Submission Energy and Specific Heat REPORT
Heat is the energy that is transferred from one body to another as a result of a difference in temperature to be able to reach thermal. What is the specific heat of a substance? What heat is needed to raise 3.4 kg of lead from 23 oc to 58 oc? Q.2 water has one of the largest specific heats of.
Solved Specific Heat Report [Physics]
Q.2 water has one of the largest specific heats of any substance. Heat is the energy transferred due to temperature differences between two different objects. The exact amount of heat q transferred due. Specific heat is unique to each substance and can be used to identify unknown substances. What is the specific heat of a substance?
Solved Energy and Specific Heat Report Sheet B. Measuring
Heat is the energy that is transferred from one body to another as a result of a difference in temperature to be able to reach thermal. What is the specific heat of a substance? Heat is the energy transferred due to temperature differences between two different objects. Calculate the specific heat capacity of a piece of wood if 1500.0 g.
Physical Science Thermal Energy Specific Heat Worksheet Answers
Equation 1 shows how to calculate specific heat. Heat is the energy transferred due to temperature differences between two different objects. Specific heat is unique to each substance and can be used to identify unknown substances. Why is this important for the human body? Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood.
Solved Exp4 (June 16, 2020) Calorimetry and Specific Heat
Heat is the energy transferred due to temperature differences between two different objects. What heat is needed to raise 3.4 kg of lead from 23 oc to 58 oc? Why is this important for the human body? Equation 1 shows how to calculate specific heat. Specific heat is unique to each substance and can be used to identify unknown substances.
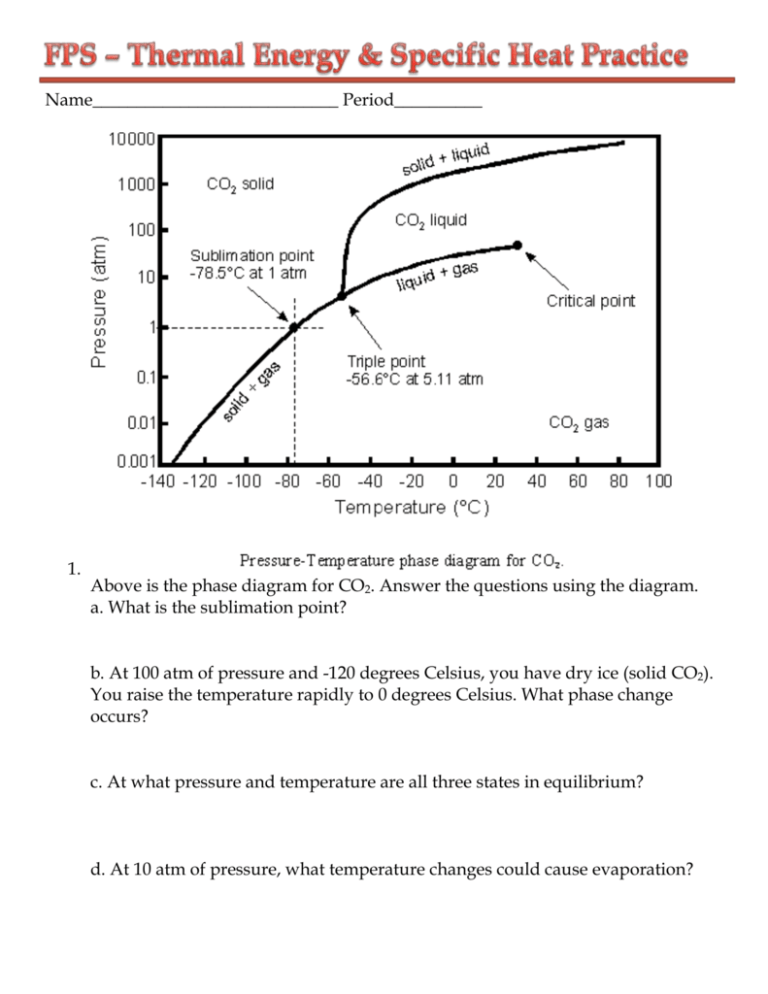
Thermal Energy & Specific Heat Practice Worksheet
What heat is needed to raise 3.4 kg of lead from 23 oc to 58 oc? Heat is the energy that is transferred from one body to another as a result of a difference in temperature to be able to reach thermal. Specific heat is unique to each substance and can be used to identify unknown substances. The exact amount.
Q.2 Water Has One Of The Largest Specific Heats Of Any Substance.
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Equation 1 shows how to calculate specific heat. Heat is the energy that is transferred from one body to another as a result of a difference in temperature to be able to reach thermal. Heat is the energy transferred due to temperature differences between two different objects.
Specific Heat Is Unique To Each Substance And Can Be Used To Identify Unknown Substances.
What heat is needed to raise 3.4 kg of lead from 23 oc to 58 oc? Why is this important for the human body? The exact amount of heat q transferred due. What is the specific heat of a substance?