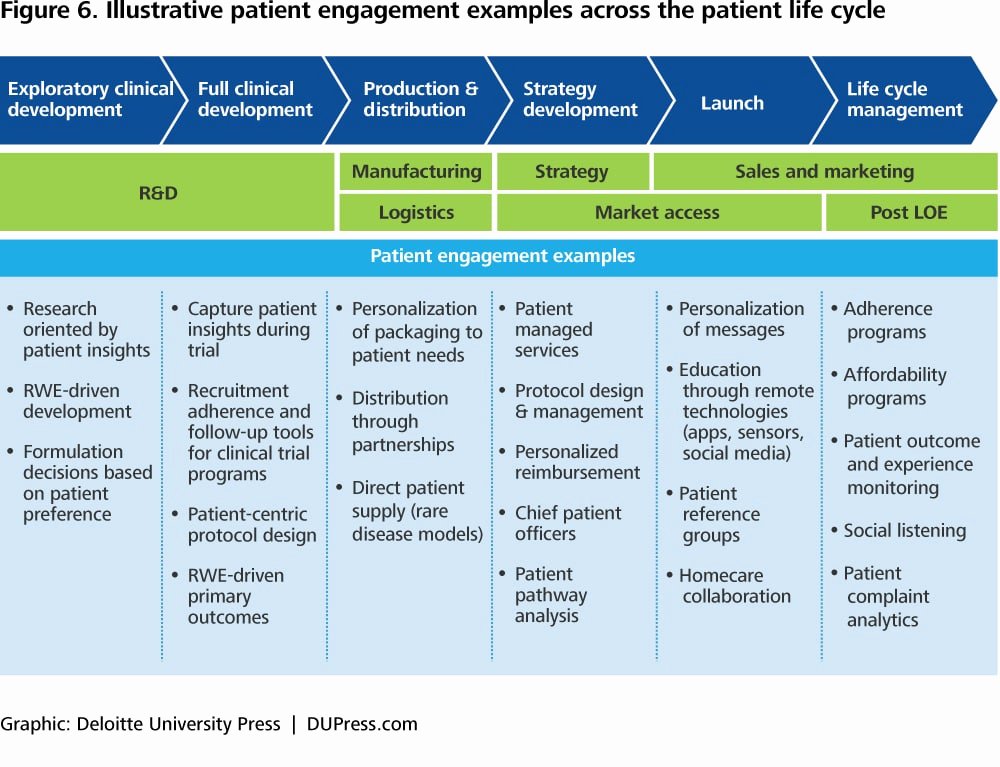
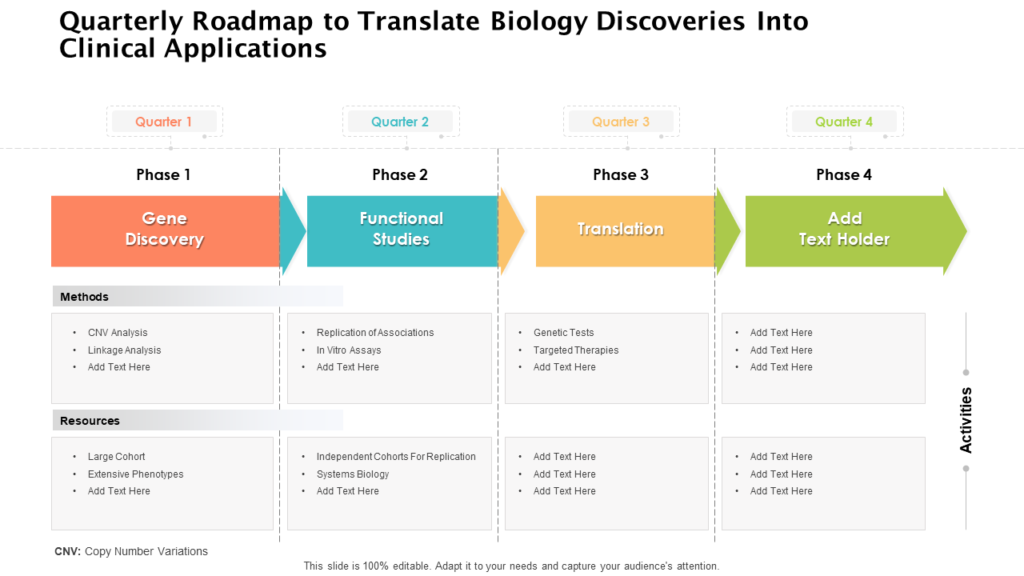
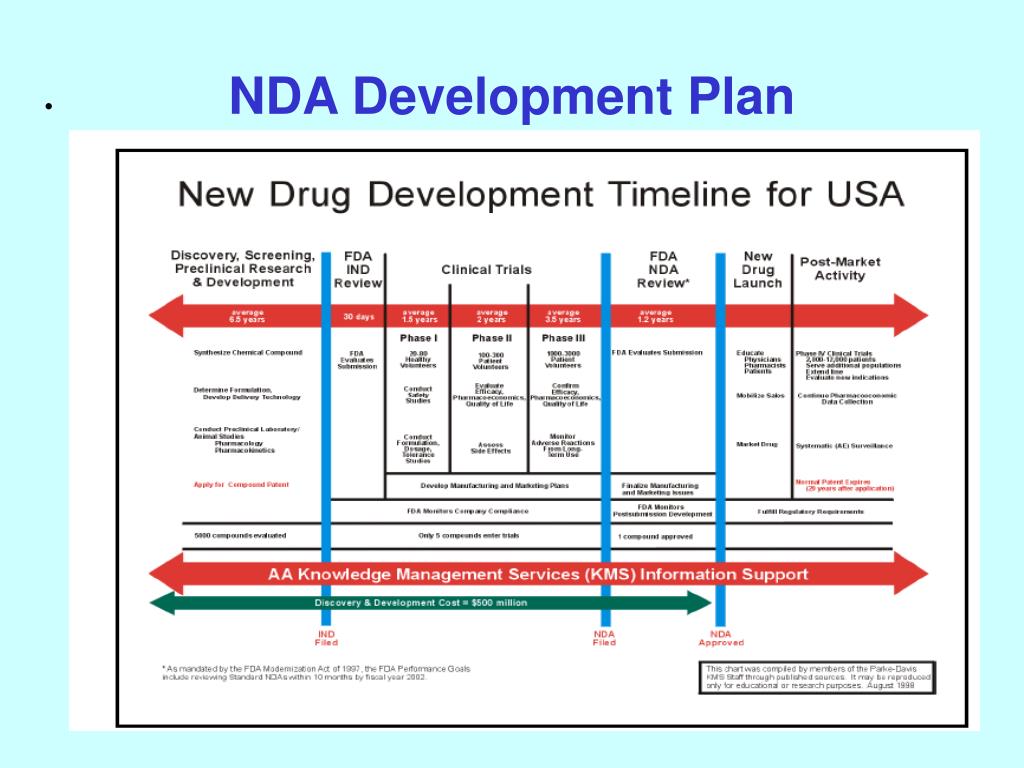
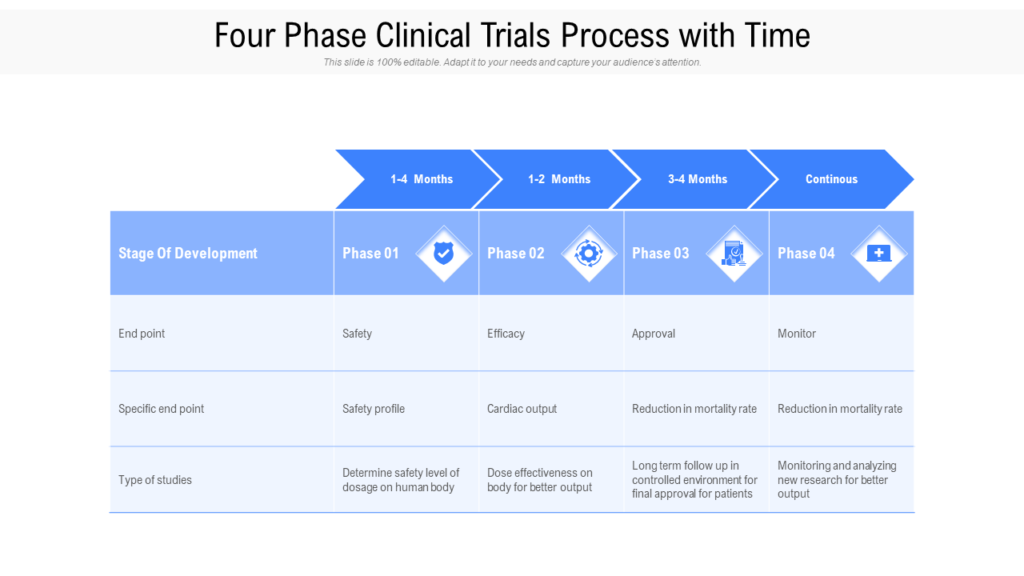
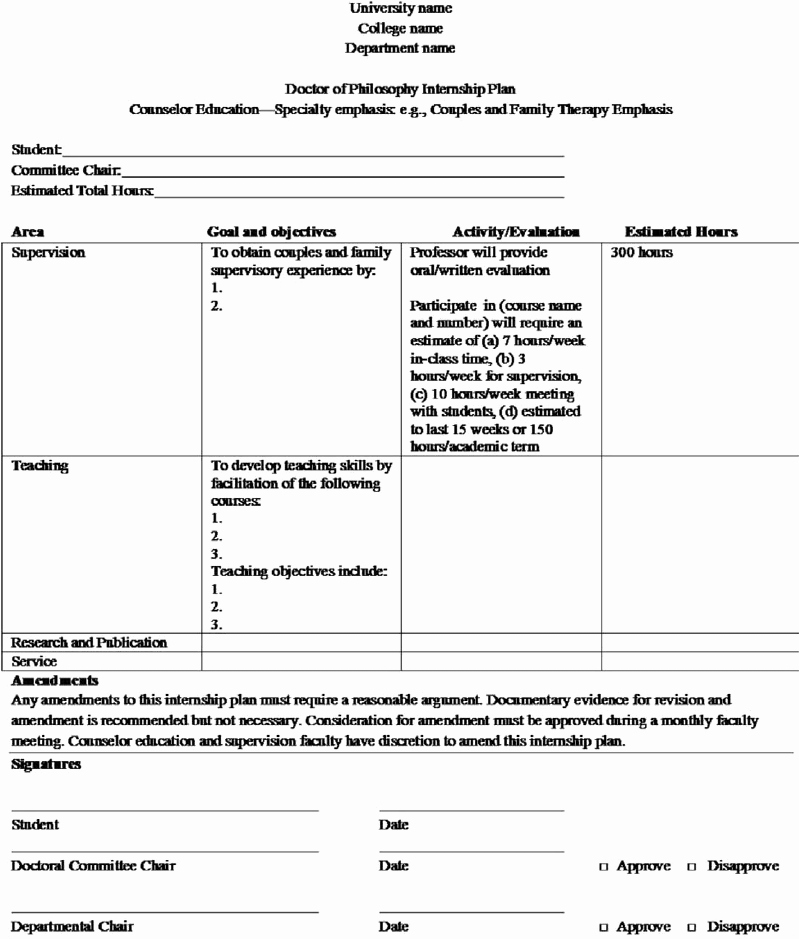
Clinical Development Plan Template Fda - The cdp should not to be confused. Guidance for industry content and format of investigational new drug applications (inds). Ind content and format for phase 1 studies. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism
The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Guidance for industry content and format of investigational new drug applications (inds). Ind content and format for phase 1 studies. The cdp should not to be confused. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism
The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Guidance for industry content and format of investigational new drug applications (inds). Ind content and format for phase 1 studies. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism The cdp should not to be confused.
Clinical Development Plan Template Fda
A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism The cdp should not to be confused. Guidance for industry content and format of investigational new drug applications (inds). The second is the clinical development plan (cdp), which can.
30 Clinical Development Plan Template Hamiltonplastering
I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. Ind content and format for phase 1 studies. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism The second is the clinical development plan (cdp), which can be viewed as the map.
Clinical Development Plan Template Fda prntbl.concejomunicipaldechinu
A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism The cdp should not to be confused. The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. Ind content.
Clinical Development Plan Template Fda
Guidance for industry content and format of investigational new drug applications (inds). I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. Ind content and.
Clinical Development Plan Template Fda
Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. Guidance for industry content and format of investigational new.
Clinical Development Plan Template
The cdp should not to be confused. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. Ind content and format for phase 1 studies. A target product profile (tpp) is a planning.
Clinical Development Plan Template Fda
Guidance for industry content and format of investigational new drug applications (inds). Ind content and format for phase 1 studies. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism I agree to comply with the protocol and ethical.
Clinical Development Plan Template Fda prntbl.concejomunicipaldechinu
Ind content and format for phase 1 studies. The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. The cdp should not to be confused. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Guidance for industry content and format.
Clinical Development Plan Template Venngage
A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. The cdp should not to be confused. I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. Guidance for industry content and format of investigational new.
Clinical Development Plan Template
I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism Ind content and format for phase 1 studies. A target product profile (tpp) is a planning tool introduced by the fda to streamline.
Guidance For Industry Content And Format Of Investigational New Drug Applications (Inds).
A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. Ind content and format for phase 1 studies. The cdp should not to be confused.
Clinical Investigational Plan Synopsis • New Onset Atrial Fibrillation • Pulmonary Embolism
I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated.